Contributors: Gary W. Rubloff, Sang Bok Lee
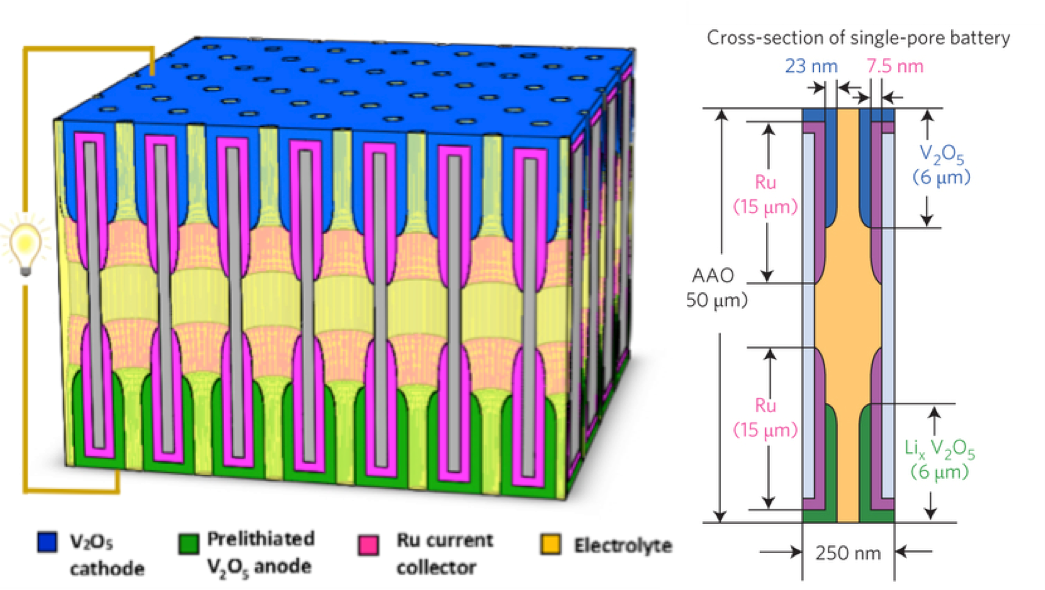
Nanostructured batteries, when properly designed and built, offer promise for delivering their energy at much higher power and longer life than conventional technology. To retain high energy density, nanostructures (such as nanowires) must be paced into dense "nanostructure forests", producing 3-D nanogeometries in which ions and electrons must rapidly move. Researchers have built arrays of nanobatteries inside billions of ordered, identical nanopores in an alumina template to determine how well ions and electrons can do their job in such ultrasmall environments. Up to a billion of these nanopore batteries could fit in a grain of sand. The nanobatteries were fabricated by atomic layer deposition to make oxide nanotubes (for ion storage) inside metal nanotubes for electron transport, all inside each end of the nanopores. The tiny nanobatteries work extremely well: they can transfer half their energy in just a 30 second charge or discharge time, and they lose only a few % of their energy storage capacity after 1000 cycles. Researchers attribute this performance to rational design and well-controlled fabrication of nanotubular electrodes to accommodate ion motion in and out and close contact between the thin nested tubes to ensure fast transport for both ions and electrons.
Complete nanobatteries are formed in each nanopore of a dense nanopore array (2 billion per cm2), using atomic layer deposition to carefully control thickness and length of multilayer concentric nanotubes as electrodes at each end.
Tiny batteries formed inside nanopores were used to demonstrate that properly scaled nanostructures can utilize the full theoretical capacity of the charge storage material while their ion insertion processes occur very fast, much like what happens at the surface of a double-layer capacitor.
These nanobatteries delivered their stored energy efficiently at high power (fast charge and discharge) and for extended cycling, demonstrating that precise nanostructures can be constructed to assess the fundamentals of ion and electron transport in nanostructures for energy storage and to test the limits of 3-D nanobattery technology.
This work was supported as part of the Nanostructures for Electrical Energy Storage (NEES), an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award number DESC0001160.