|
 |
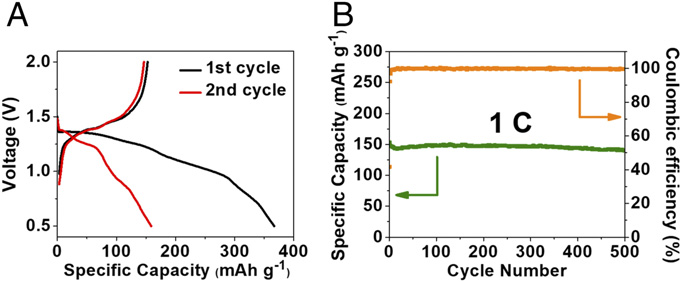
Image: The electrochemical performance of ADALS in Na-ion batteries. (A) The galvanostatic charge–discharge curves at 1 C. (B) Desodiation capacity and Coulombic efficiency versus cycle number at the current density of 1 C. |
|
Li-ion batteries (LIBs) are currently the most common batteries used in household electronics and electric vehicles. However, the use of LIBs on a mass scale has created environmental challenges, such as heavy metal and ground water pollution. Consequently, the scientific community has engaged in the development of sustainable alternative materials for LIBs, including electrode materials.
Electrode materials in LIBS are used to store ions. Some of the most common materials used to make electrodes are graphite for anodes, lithium metal oxides for cathodes. Organic electrode materials offer an attractive alternative because they are lightweight, plentiful, low-cost and eco-friendly; thus, sustainable. However, traditional organic electrodes – such as those made from organosulfur compounds, organic free-radical compounds, carbonyl (C=O) compounds and imine (C=N) compounds – suffer from poor cycle stability and low power density.
To overcome these challenges, researchers in the University of Maryland Department (UMD) of Chemical and Biomolecular Engineering (ChBE) – Drs. Chao Luo and Professor Chunsheng Wang – have developed a new cluster of organic electrode materials containing “azo functional groups” for alkali-ion batteries. Azo – coming from the French word for Nitrogen, azote – compounds bear the functional group R-N=N-R, where “R” can represent either aryl or alkyl, and “N” is nitrogen.
In this study, the azo materials utilized – azobenzene (AB), methyl red sodium salt, (MRSS) and azobenzene-4,4′-dicarboxylic acid lithium salt (ADALS) – exhibit superior electrochemical performance in Li-ion and Na-ion batteries during charge and discharge cycles.
The azo groups in the azo compounds "function as electrochemical active sites to reversibly react with Li-ion and Na-ion," said Dr. Luo. "During the reaction, the nitrogen-nitrogen double bond is reduced by Li-ion and Na-ion to generate lithiated/sodiated azo group with electron transfer. The conjugated structure in azo compounds facilitates the electron and ion transfer, while the addition of carboxylate groups in azo compounds suppresses the solubility, resulting in high-rate performance and long lifetime."
The ADALS compound specifically offers excellent electrochemical performance in both LIBs and SIBs, offering a strong materials alternative for alkali-ion batteries, regardless of application.
For additional information:
Luo, C. et al., C. Wang. “Azo compounds as a family of organic electrode materials for alkali-ion batteries.” PNAS; published online February 9, 2018. DOI: 10.1073/pnas.1717892115.
Related Articles:
Researchers’ Battery Breakthrough Improves Performance at Lower Costs
Maryland Engineers Get Cracking on Sustainability with Crab Shell-based Battery
Building Energy Innovation in Maryland
New government partner joins UMD’s Center for Research in Extreme Batteries
ARL to Fund $30M in Equipment Innovations for Service Members
University of Maryland leads team awarded $7.2M from Army Research Lab
UMD Research Team Advances the Battery Revolution
UMD researcher receives new $1M Vehicle Technology Award
Sulfur Provides Promising 'Next-Gen' Battery Alternative
UMD Researchers Design ‘Open’ Lithium-ion Battery
February 13, 2018
|